5
Electron Shells and the Bohr Model
Note that there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically neutral atoms, the number of electrons is the same as the number of protons. Thus, each element, at least when electrically neutral, has a characteristic number of electrons equal to its atomic number.
In 1913, Danish scientist Niels Bohr (1885–1962) developed an early model of the atom. The Bohr model shows the atom has a central nucleus containing protons and neutrons, with the electrons in circular orbits at specific distances from the nucleus, Figure 1. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shell. These energy levels are designated by a number and the symbol “n.” For example, 1n represents the first energy level located closest to the nucleus.
Electrons fill shells in a consistent order: they first fill the shells closest to the nucleus, then they continue to fill shells of increasing energy further from the nucleus. The electrons of the outermost energy level determine the atom’s energetic stability and its tendency to form chemical bonds with other atoms to form molecules.
Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a maximum of two electrons but the next two electron shells can each have a maximum of eight electrons. This is known as the octet rule, which states, with the exception of the innermost shell, that atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Figure 2 shows examples of some neutral atoms and their electron configurations. Notice that helium has a complete outer electron shell, with two electrons filling its first and only shell. Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one, respectively, in their outer shells, however, by following the octet rule they would be more energetically stable having eight.
VISUAL CONNECTION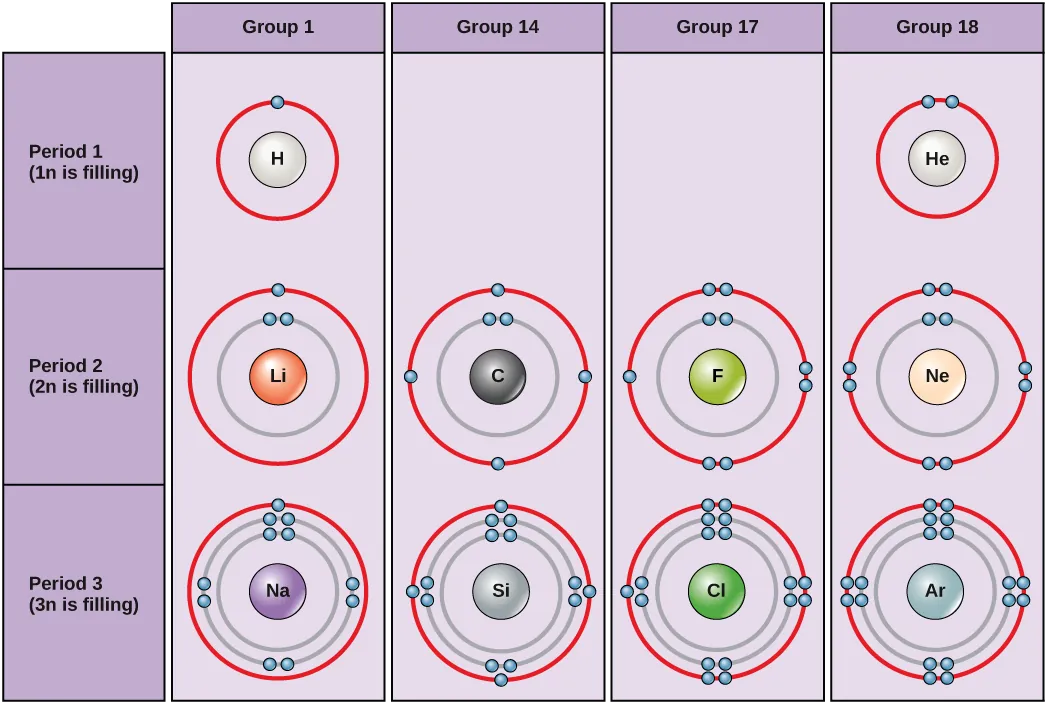
An atom may give, take or share electrons with another atom to achieve a full valence shell, the most stable electron configuration. Looking at this figure, how many electrons do elements in group 1 need to lose in order to achieve a stable electron configuration? 1 electron. How many electrons do elements in groups 14 and 17 need to gain to achieve a stable configuration? 4 and 1 electron respectively.
Understanding that the periodic table’s organization is based on the total number of protons (and electrons) helps us know how electrons distribute themselves among the energy levels. The periodic table is arranged in columns and rows based on the number of electrons and their location. Examine more closely some of the elements in the table’s far right column in Figure 3. The group 18 atoms helium (He), neon (Ne), and argon (Ar) all have filled outer electron shells, making it unnecessary for them to share electrons with other atoms to attain stability. They are highly stable as single atoms because they are nonreactive; scientists coin them inert (or noble gases). Compare this to the group 1 elements in the left-hand column. These elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. That means that they can achieve a stable configuration and a filled outer shell by donating or sharing one electron with another atom. Hydrogen will donate or share its electron to achieve this configuration, while lithium and sodium will donate their electron to become stable. As a result of losing a negatively charged electron, they become positively charged ions. Group 17 elements, including fluorine and chlorine, have seven electrons in their outermost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them to make several covalent bonds (discussed below) with other atoms. Thus, the periodic table’s columns represent the potential shared state of these elements’ outer electron shells that are responsible for their similar chemical characteristics.
octet rule
atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell, with the exception of the innermost shell
valence electrons

